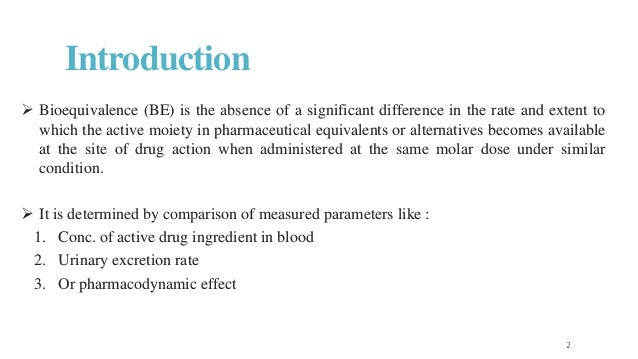
Nature of reference material and dosage form to be tested 3. In other situations bioequivalence may sometimes be demonstrated through comparative clinical trials or pharmacodynamic studies. bioequivalence study design.
Bioequivalence Study Design, Area under the plasma concentration-time curve AUC and maximum plasma concentration Cmax are the. Bioequivalence may sometimes be demonstrated using an in-vitro bioequivalence standard especially when such an in-vitro test has been correlated with human in-vivo bioavailability data. 90 CI of mean TR.
 Interchangeability And Study Design Drs Jan Welink Training Workshop Training Of Be Assessors Kiev October Ppt Download From slideplayer.com
Interchangeability And Study Design Drs Jan Welink Training Workshop Training Of Be Assessors Kiev October Ppt Download From slideplayer.com
Bioequivalence studies are often part of applications for generic veterinary medicinal products to allow bridging of safety and efficacy data associated with a reference veterinary medicinal product. Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods.
Bioequivalence studies are often part of applications for generic veterinary medicinal products to allow bridging of safety and efficacy data associated with a reference veterinary medicinal product.
Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. The test products used in the bioequivalence study must be prepared in accordance with GMP regulations. 8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Parallel design 56. This guidance document is being distributed for comment purposes only.
Another Article :

Study design Randomized twosequence twoperiod crossover Treatment sequence is randomized Every subjects gets both treatments Half get generic drug first half get brand drug first minimizesthe sequence effect Crossover design Each subject serves as hisher own control minimizes intersubject variability. Containing antacids GMWSI â Bioequivalence Study Information Form Web view in submission of study site normal values for. Objective The basic design for bioequivalence study is determined by. 8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. A bioequivalence study was carried out on two formulations of doxepin containing 15 of the active cis isomer and 85 of the less active trans isomerThe 90 confidence intervals In AUClast In Cmax and In CmaxAUClast for Ndesmethyldoxepin fell entirely within bioequivalence li whether stereoselective or non-stereoselective data were analyzed. Bioequivalence Study Design.
Taking into account the pharmacokinetic properties of Molnupiravir the following guidance with regard to the study design should be taken into account. Biometrical concepts of alternative designs and pooled analysis. Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Objective The basic design for bioequivalence study is determined by. A bioequivalence study compares the bioavailability between a test and a reference drug product in terms of the rate and extent of drug absorption. Be Bio Equivalence Study Design Basics 2 White Paper.

This study reviews the requirements of bioequivalence with study parameters such as study design fasting or fed state studies volunteers recruitment. Denote the test product and the reference product by T and R respectively. Presents the recent developments in methodology including population and individual bioequivalence. Scientific question and objectives to be answered 2. Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. Representation Of Parallel Study Design Download Scientific Diagram.
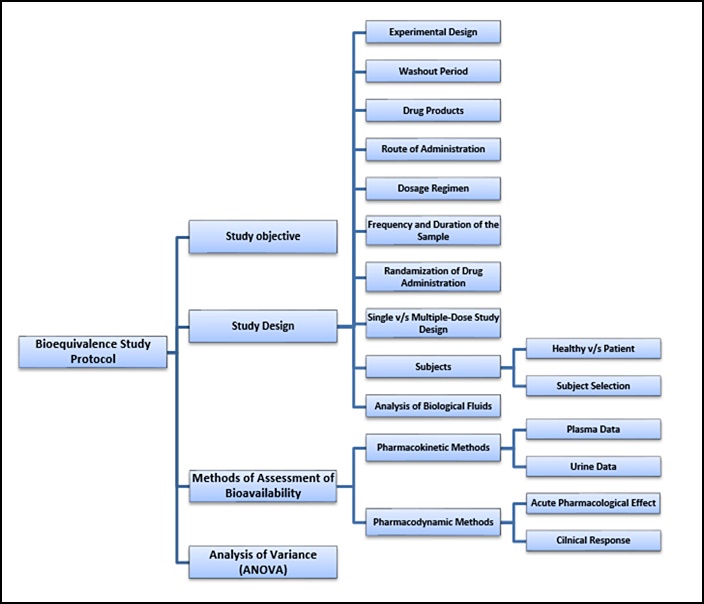
Scientific question and objectives to be answered 2. Pk and Pd of drug substance 5. Biometrical concepts of alternative designs and pooled analysis. The test products used in the bioequivalence study must be prepared in accordance with GMP regulations. Conclusion of bioequivalence studies Study design appropri ate and study conduct satisfactory No critical deficiencies or abnormalities methods or statistical analysis Bioequivalence established. Elements Of Bioequivalence Study Protocol.

The EoI includes 200 mg capsules. In a simple parallel study design the statistical analysis should be conducted including the between-subject variability to calculate the 90 confidence interval of the treatment mean difference. The EoI includes 200 mg capsules. Biometrical concepts of alternative designs and pooled analysis. Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry. Bioavilability And Bioequivalence Study Designs.

Nature of reference material and dosage form to be tested 3. Bioequivalence studies are often part of applications for generic veterinary medicinal products to allow bridging of safety and efficacy data associated with a reference veterinary medicinal product. A bioequivalence study single-dose or multi-dose should be crossover in design unless a parallel or other design is more appropriate for valid scientific reasons. Design of bioequivalence studies WHO supports applicants in addressing specific scientific issues related to product development and design of bioequivalence studies that are intended to support an application for prequalification. APIdays Paris 2019 - Innovation scale APIs as Digital Factories New Machi. Ethical Guidelines And Study Design For Bioavailability And Bioequivalence Study Semantic Scholar.
A bioequivalence study single-dose or multi-dose should be crossover in design unless a parallel or other design is more appropriate for valid scientific reasons. APIdays Paris 2019 - Innovation scale APIs as Digital Factories New Machi. In other situations bioequivalence may sometimes be demonstrated through comparative clinical trials or pharmacodynamic studies. The most common designs for bioequivalence studies are replicated crossover nonreplicated crossover and parallel. The EoI includes 200 mg capsules. Bioequivalence Study Design.
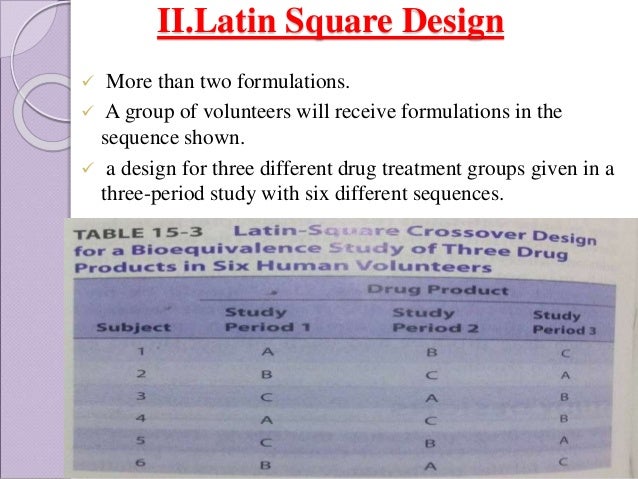
The most common designs for bioequivalence studies are replicated crossover nonreplicated crossover and parallel. 90 CI of mean TR. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. A bioequivalence study compares the bioavailability between a test and a reference drug product in terms of the rate and extent of drug absorption. Bioavilability And Bioequivalence Study Designs.

Bioequivalence studies are often part of applications for generic veterinary medicinal products to allow bridging of safety and efficacy data associated with a reference veterinary medicinal product. Taking into account the pharmacokinetic properties of Molnupiravir the following guidance with regard to the study design should be taken into account. Provides a practical overview of the design and analysis of bioequivalence studies. Design of bioequivalence studies WHO supports applicants in addressing specific scientific issues related to product development and design of bioequivalence studies that are intended to support an application for prequalification. Presents the recent developments in methodology including population and individual bioequivalence. Design Of The Four Bioequivalence Studies Download Scientific Diagram.

Bioequivalence - Study Design Considerations Study Design Considerations. Presents the recent developments in methodology including population and individual bioequivalence. Objective The basic design for bioequivalence study is determined by. Provides a practical overview of the design and analysis of bioequivalence studies. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. Study Design For The Bioequivalence Evaluation Of Test And Reference Download Table.

The availability of analytical methods 4. In bioequivalence studies the study design also determines the appropriate statistical model for data analysis. Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Scientific question and objectives to be answered 2. Provides a practical overview of the design and analysis of bioequivalence studies. Bioavailability And Bioequivalence Study Designs Youtube.

A bioequivalence study single-dose or multi-dose should be crossover in design unless a parallel or other design is more appropriate for valid scientific reasons. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. Area under the plasma concentration-time curve AUC and maximum plasma concentration Cmax are the. Containing antacids GMWSI â Bioequivalence Study Information Form Web view in submission of study site normal values for. Bioequivalence - Study Design Considerations Study Design Considerations. Bioavilability And Bioequivalence Study Designs.

Taking into account the pharmacokinetic properties of Molnupiravir the following guidance with regard to the study design should be taken into account. 90 CI of mean TR. Area under the plasma concentration-time curve AUC and maximum plasma concentration Cmax are the. Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Design of bioequivalence studies WHO supports applicants in addressing specific scientific issues related to product development and design of bioequivalence studies that are intended to support an application for prequalification. Bioavailability And Bioequivalence Ppt Video Online Download.

As recommended by the USFDA in most bioequivalence studies a test drug is compared with the standard reference drug in a group of normal healthy subjects of age 1855 years each receives both the treatments alternately in a crossover fashion two-period two-treatment crossover design with the two phases of treatment separated by a washout period. This study reviews the requirements of bioequivalence with study parameters such as study design fasting or fed state studies volunteers recruitment. Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. An overview of the generic drug approval process Division of Bioequivalence II Reviewer Kimberly W. Bioequivalence Study Design.

Objective The basic design for bioequivalence study is determined by. Area under the plasma concentration-time curve AUC and maximum plasma concentration Cmax are the. Bioequivalence studies are often part of applications for generic veterinary medicinal products to allow bridging of safety and efficacy data associated with a reference veterinary medicinal product. A single-dose cross-over design is recommended. The EoI includes 200 mg capsules. Study Design Of The Crossover Bioequivalence Study Download Table.










